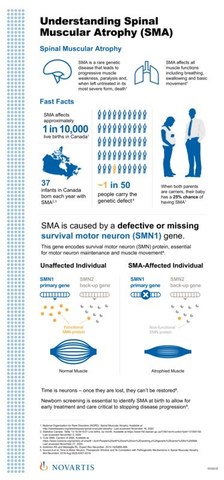
Zolgensma® data including patients with more severe SMA at baseline further demonstrate therapeutic benefit, including prolonge

Novartis' Zolgensma expansion hits FDA roadblock, giving Biogen and Roche a reprieve | Fierce Pharma

R&D report card: Zolgensma-maker Novartis' spending reaches $9.4B in 2019 | S&P Global Market Intelligence
FDA Lifts Partial Clinical Hold on Novartis' Gene Therapy Trial for Spinal Muscular Atrophy | BioSpace
Zolgensma® data shows rapid, significant, clinically meaningful benefit in SMA including prolonged event- free survival, motor

Zolgensma® Data Shows Rapid, Significant, Clinically Meaningful Benefit in SMA, Including Prolonged Event-free Survival, Motor Milestone Achievement and Durability, Now Up to 5 Years Post-dosing - Cure SMA